Chemistry Manufacturing & Controls
CMC is a term used when drug developers define their investigative drug substance, establish manufacturing methods for that drug, and develop a strategy for controlling the quality and stability of the product from early phases through post-approval production.
CMC STRATEGY
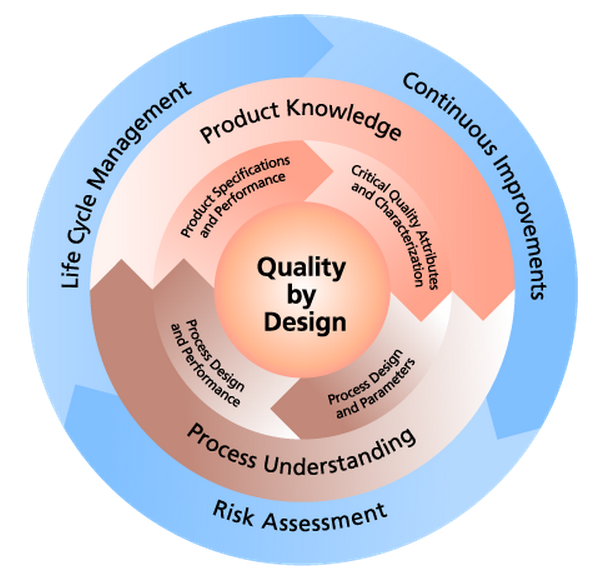
A CMC strategy is a science-based plan for ensuring a product’s quality and safety throughout development. It is often tailored to the specific product, its delivery method, and its development timeline. It’s also important to engage with regulatory authorities to ensure that the strategy meets their expectations.
Elements of a CMC Strategy:
- Quality attributes: Identifying characteristics that must be monitored, such as safety, purity, and strength
- Analytical methods: Developing methods to measure quality attributes
- Manufacturing processes: Optimizing processes to ensure consistent production
- Supply chain controls: Implementing controls to ensure consistent supply
- Risk assessment: Identifying and managing potential risks
- Regulatory compliance: Ensuring compliance with regulatory requirements
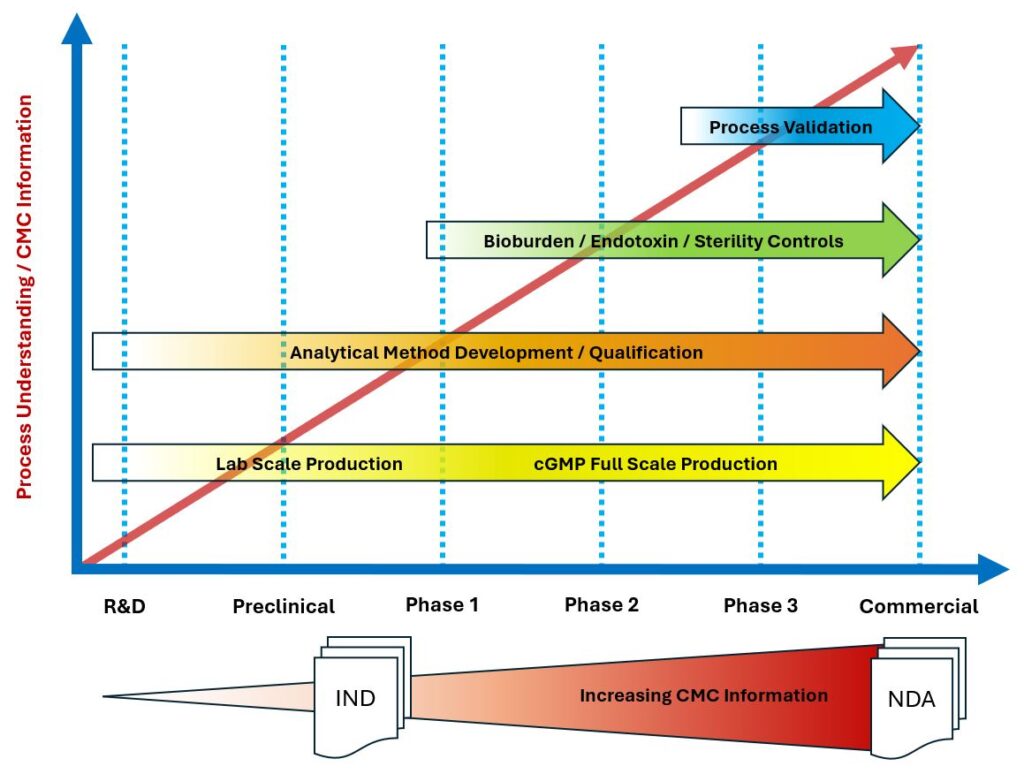
Understanding the regulatory requirements for CMC information for an Investigational New Drug (IND) application and for a New Drug Application (NDA) will be an important factor for the development of a CMC Strategy.
Throughout the development process CMC information is collected and analyzed, resulting in increased product and process understanding. The CMC information required for an IND application often leverages existing data to establish the identification, quality, purity and strength of the investigational drug product for a phase 1 study, while the CMC information required for an NDA statistically demonstrates the specific controls for the product and process at full scale commercial production.
As more CMC information is collected throughout the development process, the CMC Strategy may change, based on the increased product and process understanding.
IND CMC REQUIREMENTS
Drug Substance
A description of the drug substance, including its physical, chemical, or biological characteristics; the name and address of its manufacturer; the general method of preparation of the drug substance; the acceptable limits and analytical methods used to assure the identity, strength, quality, and purity of the drug substance; and information sufficient to support stability of the drug substance during the toxicological studies and the planned clinical studies. Reference to the current edition of the United States Pharmacopeia—National Formulary may satisfy relevant requirements in this paragraph.
Drug Product
A list of all components, which may include reasonable alternatives for inactive compounds, used in the manufacture of the investigational drug product, including both those components intended to appear in the drug product and those which may not appear but which are used in the manufacturing process, and, where applicable, the quantitative composition of the investigational drug product, including any reasonable variations that may be expected during the investigational stage; the name and address of the drug product manufacturer; a brief general description of the manufacturing and packaging procedure as appropriate for the product; the acceptable limits and analytical methods used to assure the identity, strength, quality, and purity of the drug product; and information sufficient to assure the product’s stability during the planned clinical studies. Reference to the current edition of the United States Pharmacopeia—National Formulary may satisfy certain requirements in this paragraph.
Placebo
A brief general description of the composition, manufacture, and control of any placebo used in a controlled clinical trial.
Labeling
A copy of all labels and labeling to be provided to each investigator.
Environmental analysis requirements
A claim for categorical exclusion under 21 CFR Part 25, § 25.30 or 25.31 or an environmental assessment under § 25.40.
NDA CMC REQUIREMENTS
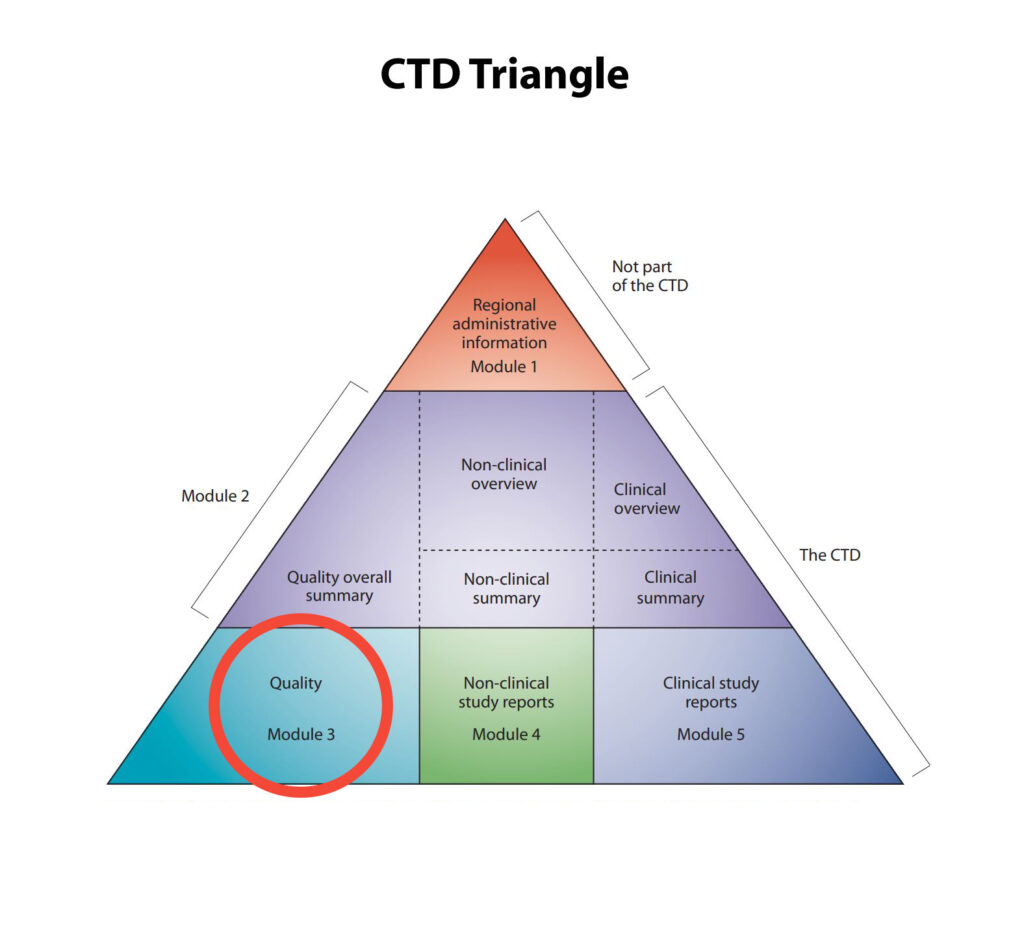
eCTD Module 3, Quality
- Drug Substance (3.2.S)
- General Information
- Manufacture
- Characterization
- Control of Drug Substance
- Reference Standards
- Container Closure System
- Stability
- Drug Product (3.2.P)
- Description and Composition of Drug Product
- Pharmaceutical Development
- Manufacture
- Control of Excipients
- Control of Drug Product
- Reference Standards or Materials
- Container Closure System
- Stability
- Appendices (3.2.A)
- Facilities and Equipment
- Adventitious Agents Safety Evaluation
- Excipients
- Regional Information (3.2.R)
- Executed Batch Records (US)
- Method Validation Package (US)
- Comparability Protocols (US)
- Process Validation Scheme for the Drug Product (EU)
- Medical Device (EU)