Pharmaceutical Development
TYPES OF DRUG APPLICATIONS
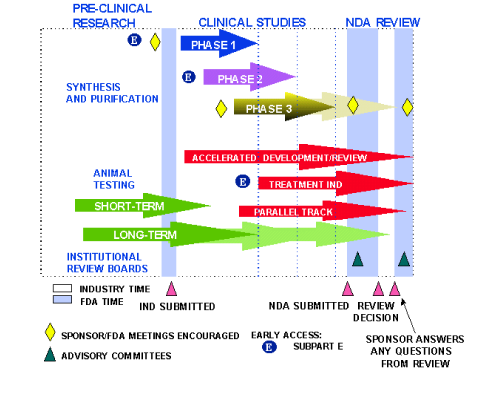
- Investigational New Drug (IND)
Current Federal law requires that a drug be the subject of an approved marketing application before it is transported or distributed across state lines. Because a sponsor will probably want to ship the investigational drug to clinical investigators in many states, it must seek an exemption from that legal requirement. The IND is the means through which the sponsor technically obtains this exemption from the FDA. - New Drug Application (NDA)
When the sponsor of a new drug believes that enough evidence on the drug’s safety and effectiveness has been obtained to meet FDA’s requirements for marketing approval, the sponsor submits to FDA a new drug application (NDA). The application must contain data from specific technical viewpoints for review, including chemistry, pharmacology, medical, biopharmaceutics, and statistics. If the NDA is approved, the product may be marketed in the United States. For internal tracking purposes, all NDA’s are assigned an NDA number. - Abbreviated New Drug Application (ANDA)
An Abbreviated New Drug Application (ANDA) contains data that, when submitted to FDA’s Center for Drug Evaluation and Research, Office of Generic Drugs, provides for the review and ultimate approval of a generic drug product. Generic drug applications are called “abbreviated” because they are generally not required to include preclinical (animal) and clinical (human) data to establish safety and effectiveness. Instead, a generic applicant must scientifically demonstrate that its product is bioequivalent (i.e., performs in the same manner as the innovator drug). Once approved, an applicant may manufacture and market the generic drug product to provide a safe, effective, low cost alternative to the American public. - Biologic License Application (BLA)
Biological products are approved for marketing under the provisions of the Public Health Service (PHS) Act. The Act requires a firm who manufactures a biologic for sale in interstate commerce to hold a license for the product. A biologics license application is a submission that contains specific information on the manufacturing processes, chemistry, pharmacology, clinical pharmacology and the medical affects of the biologic product. If the information provided meets FDA requirements, the application is approved and a license is issued allowing the firm to market the product.
DOSAGE FORMS & ROUTE OF ADMINISTRATION
Dosage forms (also called unit doses) are pharmaceutical drug products presented in a specific form for use. They contain a mixture of active ingredients and inactive components (excipients), configured in a particular way and apportioned into a specific dose.
Dosage forms vary depending on the method and route of administration, which can include many types of liquid, solid, and semisolid forms. Common dosage forms include tablets, capsules, syrups, creams, and injectables.
A combination drug (or fixed-dose combination; FDC) is a product that contains more than one active ingredient.
The route of administration (ROA) for drug delivery depends on the dosage form of the substance. Different dosage forms may be available for a particular drug, especially if certain conditions restrict the ROA. A specific dosage form may also be required due to issues such as chemical stability or pharmacokinetic properties.
CHOOSING A DOSAGE FORM
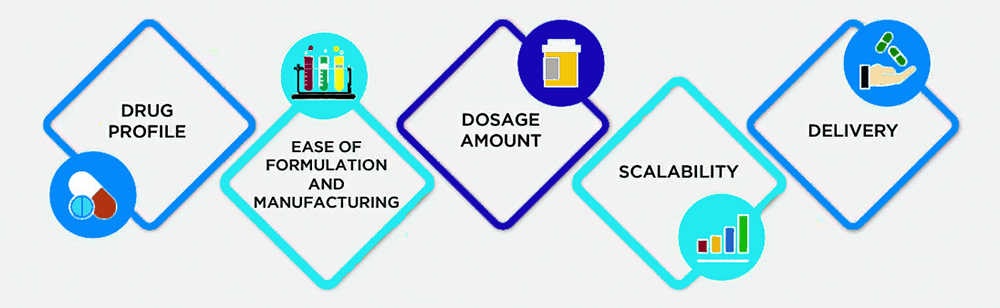
The proper design and formulation of a dosage form requires consideration of the physical, chemical, and biological characteristics of all of the drug substances (active pharmaceutical ingredients (APIs)) and pharmaceutical ingredients (excipients) to be used in manufacturing the drug product. The API and excipients utilized must be compatible and produce a drug product that is stable, efficacious, palatable, easy to administer, and well tolerated. Formulation factors include physical properties such as particle size, crystalline structure, melting point, solubility, partition coefficient, dissolution, membrane permeability, dissociation constants, and drug stability. Successful development of a dosage form includes multiple considerations involving the drug, excipients, compliance, storage, packaging, and stability, as well as patient considerations of taste, appearance, and palatability.
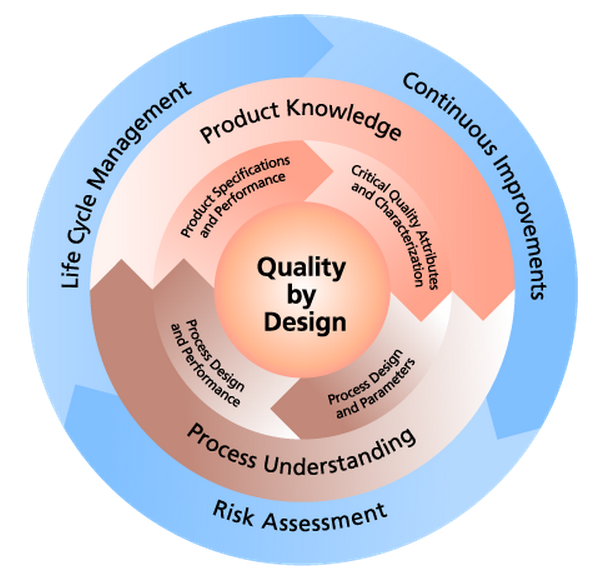
CHEMISTRY MANUFACTURING & CONTROLS
Chemistry Manufacturing & Controls (CMC) is an essential part of the Drug Development Process. CMC is a term used when drug developers define their investigative drug substance, establish manufacturing methods for that drug, and develop a strategy for controlling the quality and stability of the product from early phases through post-approval production.